 With older on-premises (single-tenant) QMS solutions, software validation is more challenging because each and every customer uses a unique instance of the software application and supporting infrastructure which must be installed, tested, and validated.
With older on-premises (single-tenant) QMS solutions, software validation is more challenging because each and every customer uses a unique instance of the software application and supporting infrastructure which must be installed, tested, and validated.
Arena’s QMS is a cloud-based, multitenant solution, architected to provide customer-requested enhancements and value-added new features on a regular schedule. With a level of shared infrastructure for all QMS customers, validation is made easier by leveraging common test protocols for both installation qualification (IQ) and operational qualification (OQ).
Arena Validate is available to all Arena QMS customers. It is supported by a dedicated validation team, allowing Arena to do most of the heavy lifting. This allows customers to focus on reviewing Arena’s validation scope and documentation and determining the customer-specific intended uses (required to handle performance qualification or PQ) that will supplement the validation package.
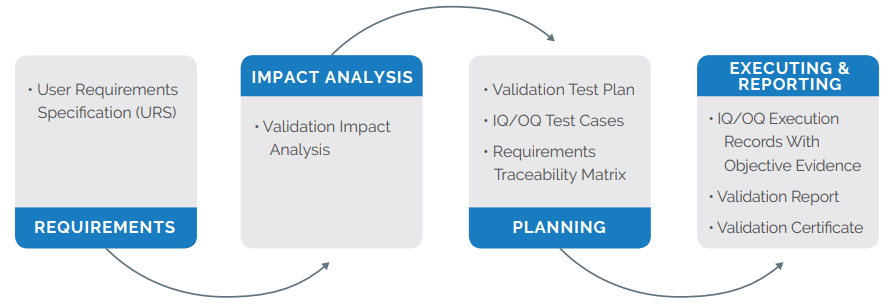
Arena Validate provides key deliverables to reduce traditional burdens on medical device companies and quality assurance regulatory affairs stakeholders.
With Arena Validate, customers get a simplified validation experience that reduces manual efforts and time-consuming work. Removing traditional validation barriers enables companies to keep pace with the latest technological and functional features and frees quality and regulatory resources to focus on innovation, market responsiveness, and continual improvement to deliver high-quality products.

According to a study by LNS Research, software validation is one of the top challenges in speeding products from R&D to market for life science companies. Furthermore, they explain that cloud-based technologies are a key differentiator to provide streamlined validation.
Arena Validate leverages the Cloud to reduce medical device manufacturers’ validation efforts and overhead. The solution’s templated and standardized approach provides validation for every software release against pre-defined common requirements to help Arena’s QMS customers address validation regulation with confidence. Customers report significant cost and time savings associated with ongoing software validation plus a renewed focus on core competencies.
Gone are the days when medical device manufacturers can stick their heads in the sand and hope their software solutions keep up with their evolving new product introduction (NPI) and quality management needs. To maintain a competitive advantage in the global market, companies can turn to Arena QMS to gain the control, compliance, and product development benefits necessary to stay ahead of the pack. Best of all, Arena Validate will ensure that quality and product teams can focus on delivering high-quality, safe, and compliant products to market fast.
To see firsthand how Arena Validate can solve your software validation struggles, request a demo today.