A device master record (DMR) is a collection of records that contains the procedures and specifications for a finished medical device. According to the FDA quality system regulation, a device master record should include, or refer to the location of, the following items:
(a) Device specifications including appropriate drawings, composition, formulation, component specifications, and software specifications
(b) Production process specifications including the appropriate equipment specifications, production methods, production procedures, and production environment specifications
(c) Quality assurance procedures and specifications including acceptance criteria and the quality assurance equipment to be used
(d) Packaging and labeling specifications, including methods and processes used
(e) Installation, maintenance, and servicing procedures and methods
Source: https://www.accessdata.fda.gov
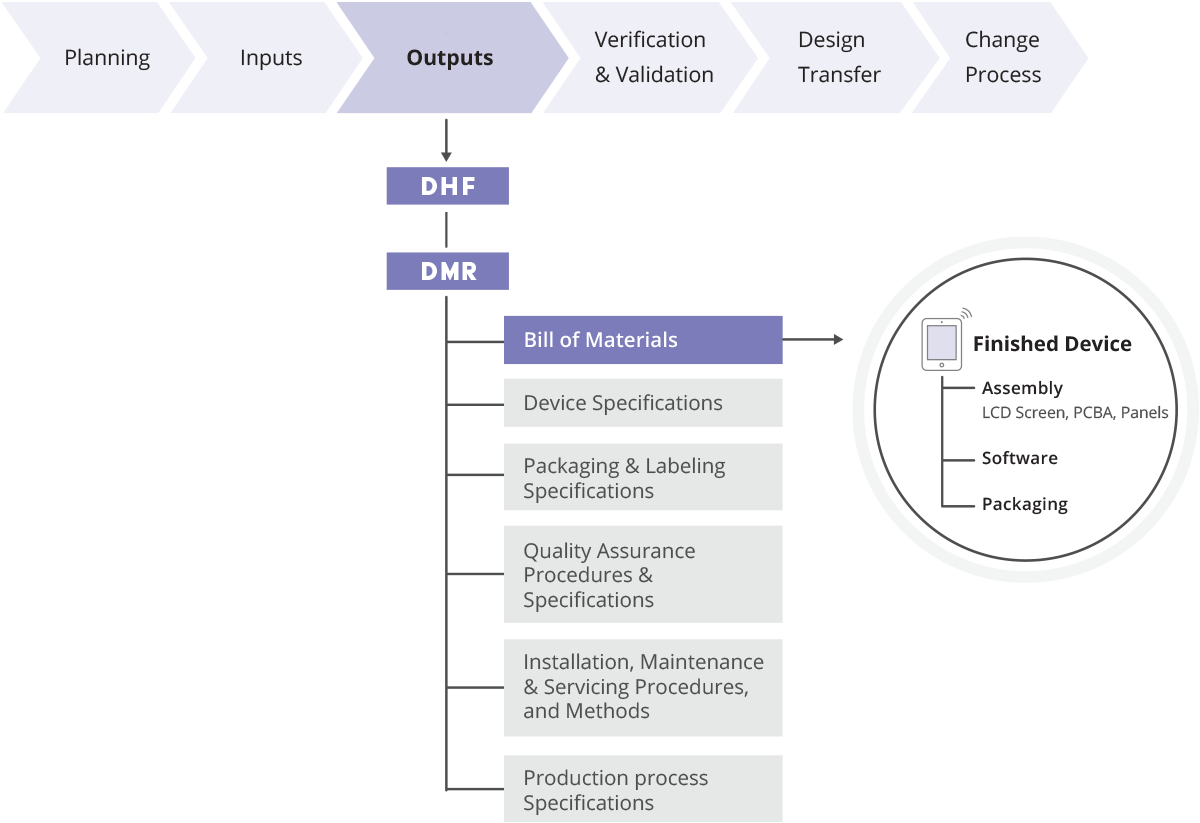
The design history file is a collection of records that describes the design history of a finished product, whereas the device master record is a collection of drawings, instructions, and other records that are needed to build a finished product.
The device master record must include:
All records that are required to build a finished product from start to finish goes into the device master record. This includes:
Learn how to manage and maintain the device master record (DMR) to ensure compliance with FDA 21 CFR part 11 and part 820.
