From Requirements to Launch: Arena’s Advanced QMS Capabilities Help Speed Innovation
 Getting high-quality, compliant products to market is a long and arduous journey for most manufacturers. In the case of medical device companies, it takes three to seven years to introduce new products. While much of this time is dedicated to conceptualization, prototyping, and testing, the approval process by regulatory bodies such as the Food and Drug Administration (FDA) and European Commission (e.g., 510(k), premarket approval, CE certification) can take up to one year. Ultimately, the timeline will vary based on the device’s intended use, risk, and classification.
Getting high-quality, compliant products to market is a long and arduous journey for most manufacturers. In the case of medical device companies, it takes three to seven years to introduce new products. While much of this time is dedicated to conceptualization, prototyping, and testing, the approval process by regulatory bodies such as the Food and Drug Administration (FDA) and European Commission (e.g., 510(k), premarket approval, CE certification) can take up to one year. Ultimately, the timeline will vary based on the device’s intended use, risk, and classification.
Companies in other industries like high-tech electronics, aerospace and defense, and clean tech have their own unique set of quality, environmental, and/or security regulations which also significantly impact their product development schedules.
Regardless of the business, today’s manufacturers are under pressure to innovate faster and gain first-mover advantages while operating under the constraints of stringent regulatory requirements and greater product complexity.
Here, we review the three major challenges that impede companies from meeting aggressive launch deliverables and gaining a competitive advantage. We also reveal how Arena’s advanced quality management capabilities help organizations overcome these setbacks to introduce products faster.
3 KEY PRODUCT DEVELOPMENT CHALLENGES FACING TODAY’S MANUFACTURER
1. Product Requirements Management
The ability to effectively manage and track requirements throughout the entire new product development process is critical for manufacturers.
Under Title 21 CFR Part 820 (c), the FDA specifies, “Each manufacturer shall establish and maintain procedures to ensure that the design requirements relating to a device are appropriate and address the intended use of the device, including the needs of the user and patient. The procedures shall include a mechanism for addressing incomplete, ambiguous, or conflicting requirements. The design input requirements shall be documented and shall be reviewed and approved by a designated individual(s).”
To demonstrate compliance, cross-functional teams must regularly document, analyze, trace, prioritize, and agree on requirements. Additionally, changes must be controlled and communicated to relevant stakeholders.
Without a unified, connected, and collaborative system to manage requirements, companies cannot ensure product performance and meet quality demands. According to LNS Research, over 90% of organizations use spreadsheets and electronic documents to track their product requirements1. And a survey by the 280 Group revealed that teams within 49.2% of organizations use different processes to manage requirements2.
Because teams are often using disparate systems that are not connected to the product record, it is difficult to adequately address all the necessary requirements and keep everyone aligned.
2. Manual, Time-Consuming Processes
To meet FDA, ISO, and other regulatory requirements, companies must document and maintain extensive compliance evidence. This includes standard operation procedures (SOPs), corrective action reports (CARs), non-conforming materials reports (NCMRs), product requirements, design history files (DHFs), device master records (DMRs), and more.
With conventional office tools such as Excel and Word, keeping documentation up to date is a painstaking task. When a particular quality process is revised and approved, it often necessitates manual updates to other related processes and records so that full traceability is maintained. This manual, disconnected approach becomes too laborious, not to mention problematic. It leads to inconsistencies and errors which pose a compliance risk.
3. Supply Chain Disruptions
Since COVID, supply chain issues continue to negatively impact manufacturing. According to a recent Deloitte survey3, ongoing supply chain disruptions cut profits for manufacturing companies by as much as 13%. Factors such as an increase in raw material pricing, part shortages, and a lack of alternate sources have led to delays in production. Whether it be due to pandemics, inflation, regional conflicts, or natural disasters, supply chain disruptions will persist in the years to come. Ultimately, manufacturers will need to embrace modern digital solutions that drive greater transparency and visibility across their supply chain.
ARENA ENHANCES TEAM ALIGNMENT AND AUTOMATES QUALITY PROCESSES
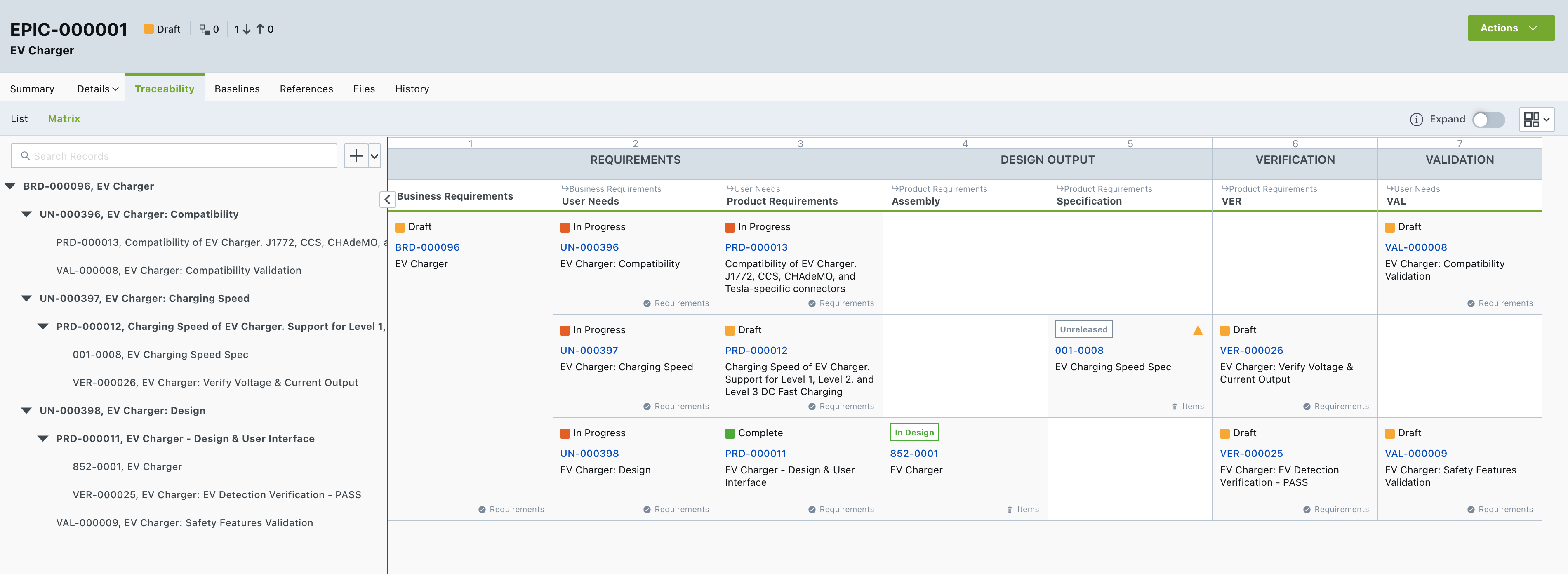
Requirements Traceability Matrix Improves Product Management
Arena’s Traceability Matrix is designed to help teams better manage product requirements and track their progress. It also provides a systematic way to trace and ensure that all requirements are addressed. With increased visibility into your product’s overarching requirements, cross-functional teams can manage their projects more effectively.
Additional benefits of the Traceability Matrix include:
- Cross-functional team alignment
- Facilitation of reviews for missing features
- Priority alignment for resources
- Test coverage and tracking
- User documentation coverage and tracking
- Project tracking
Automations Streamlines Quality Processes
The new Automations functionality within Arena enables you to place routine quality processes into an automation workflow. For example, a user can easily configure Arena to automatically generate a corrective action request once a non-conformance material request has been approved. The ability to automate these types of repetitive tasks shortens process cycle times significantly and minimizes manual data errors.
ARENA’S CLOUD-NATIVE PLATFORM DRIVES SUPPLY CHAIN TRANSPARENCY AND VISIBILITY
Arena’s cloud-native platform provides a secure, unified system for product teams and supply chain partners to collaborate regularly on all things product and quality related. By eliminating data silos and communication gaps, everyone can gain visibility into operational issues early on and mitigate supply chain risks.
EXPLORE ARENA QMS
Request a demo to experience all that Arena QMS has to offer.
References
- LNS Research. Modern Approach to New Product Introduction (NPI). 2018.
- 280 Group. Challenges in Product Management Survey Results. 2015.
- Deloitte. Meeting the Challenge of Supply Chain Disruption. 2022.