Arena Validate: IQ and OQ Confidence
 Arena’s life sciences customers recently attended a virtual event with experts on Arena Validate. We all learned something from this exciting session. Read the FDA Software Validation eBook.
Arena’s life sciences customers recently attended a virtual event with experts on Arena Validate. We all learned something from this exciting session. Read the FDA Software Validation eBook.
What Is the Validate Subscription?
Arena Validate helps life sciences companies achieve and maintain regulatory compliance by providing ongoing Arena application validation maintenance.
For an Arena Insider’s look at Validate, check out this blog.
Benefits for Life Sciences Customers
Early in Arena’s life, we discovered that our multi-tenant cloud architecture and configurable applications together enabled us to significantly help customers’ software validation efforts. By integrating the validation process into the software release process in-house, we can offer a unique value proposition to life sciences companies.
Life sciences customers subscribe to Validate for many reasons. Some have had experience validating other enterprise systems, so have suffered the consequences of DIY. They see how Arena Validate dramatically reduces the overall time and effort needed to validate Arena per intended use. Others like that Validate frees up more time so their validation team can thoroughly evaluate the impact of each new release. Another major reason to subscribe is to use the latest, current release with confidence, knowing that it is has been validated.
Many life sciences employees have experienced “revision lock” on old hardware, operation systems, or applications because it is the validated revision. This is frustrating! Your company pays annual software maintenance fees, yet you cannot use the newest features and fixes because validating a new revision is too expensive and time-consuming. Finally, Arena Validate subscribers are confident that when an auditor arrives, their installation qualification (IQ) and operational qualification (OQ) are completely documented and easily accessible—even if it is a remote audit!Arena also provides performance qualification (PQ) templates to assist Validate customers in determining their PQ validation needs, if any.
Software Validation Process
The Arena Validate process is integrated with the Arena product release process. Arena Validate tests the Arena application against predefined intended uses. An Arena release cannot go live until it has been validated.
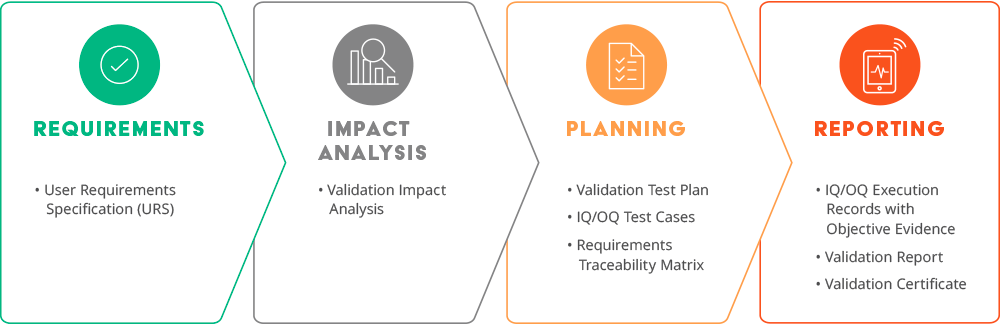
The predefined and approved validation requirements are designed to cover use cases applicable to most customers. These user requirements have grown significantly over time—from 86 requirements in 2008 to over 1,600 requirements in 2023! The three main reasons for this growth are upgrades to existing validated functionality, internal and external feedback, and new Arena products. As a Validate subscriber, if you do not see a specific intended use, let us know because we may add it to the validation scope, which benefits all subscribers.
A User Requirements Specification and an Impact Analysis are the first document set we deliver before each major release. Each customer’s validation expert uses this information to determine the risk to their implementation and the impact on their standard operating procedures (SOPs). They can mitigate the risk by testing their unique intended uses. Examples of unique intended uses are custom workflows or settings that are not Validate intended uses as well as integrations. Finally, life sciences companies sometimes must revise their SOP documents to keep them in sync with the current Arena release.
To complete the documentation, we deliver two more document sets—one set before the software release, and another following go live. Just before the software release, we provide Validate subscribers: traceability matrices, OQ/IQ1 protocol execution records, a software release test plan, and a report summary containing all prerelease Arena Validate activities.
Following a successful software release, we execute the required post-deployment testing (IQ2), which is then shared with Validate subscribers along with an updated report summary and a validation certificate, concluding Validate documentation delivery for the release.
Watch this overview of the documentation provided in our validation package.
Is Arena 21 CFR Part 11 Compliant?
We had a spirited discussion during the question-and-answer part of the webinar. The top question asked was about Arena 21 CFR Part 11 compliance.
The FDA’s 21 CFR Part 11 regulation governs electronic signatures for life sciences companies. The short answer is that Arena provides the technical controls necessary to perform electronic signatures and meet 21 Part 11 compliance. You need to implement compliant practices as well as the technology. This best practice resource reviews five areas to consider when setting up compliant electronic signatures.
Software validation can be challenging and time-consuming. Whether you’re an existing Arena QMS life sciences customer or are considering QMS solutions, it’s important to understand the validation process. Arena Validate helps speed validation and meet FDA 21 CFR Part 820 and Part 11 compliance.
For more information, read: FDA Software Validation: How Cloud QMS Reduces Costs and Resource Drains


